Homogeneous
Catalysis
We are
studying catalysts which will give very high selectivity to
desirable products often via cascade reactions. In Scheme 1,
α,ω-diesters are formed in a single cascade reaction by the
methoxycarbonylation of alkynes. The same catalyst catalyses the
methoxy-carbonylation to the α, Β-unsaturated ester (the reaction can
be stopped here), the double bond isomerisation and the second
methoxycarbonyation, which only occurs when the double bond is in the
least thermodynamically favoured terminal position.1
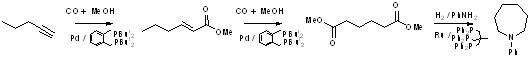
Scheme 1. Formation of dimethyl adipate
from 1- butyne by a
methoxycarbonylation - isomerisation - isomerisation Cascade sequence;
also shown is the reductive amination of dimethyl adipate to
N-phenylazacycloheptane
In a
separate reaction, also shown in Scheme 1, α,ω-diesters can be
converted by reductive amination to heterocycles. Related reactions are
the formation of long chain α,ω-diesters from natural feedstocks
(Scheme 2)2
and the catalytic hydrogenation of amides to amines (Scheme
3).3
Scheme 2. Upgrading of methyl oleate to a
polymer precursor
|
Scheme 3. Hydrogenation of amides to amines
|
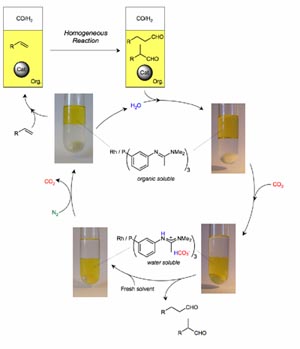
In
addition, we have a major programme on new approaches to separating
products from the catalyst in homogeneous reactions using biphasic
systems involving aqueous, ionic liquid and supercritical fluid
solvents. Recent studies have included additives to aqueous biphasic
systems which give rate enhancements of 100 times without catalyst
leaching or emulsion formation, catalysts which can be switched between
water and organic phases by bubbling or removing CO2 (Figure
1) and supported ionic liquid phase catalysts with supercritical flow.4
Figure 1.
Catalyst separation by transfer
into and from water using CO2
|
 |
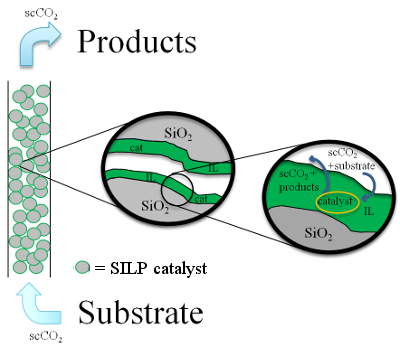 Supported
ionic liquid phase catalysts are composed of a thin layer of ionic
liquid adsorbed onto the support pores, being the catalyst dissolved in
the ionic liquid. This type of catalyst has been used to perform
different reactions in a continuous flow mode with supercritical CO 2
as
carrier gas. The substrates, fed continuously, are dissolved into the
supercritical CO 2
and diffused into the ionic liquid layer to react with the catalyst and
the products being also removed by the stream of
supercritical CO 2. The ionic liquid is
insoluble in CO 2 avoiding leaching of
it and the catalyst (Figure 2).
Figure 2. Squeme of continuous flow
SILP catalysis
This system has been succesfully applied to different reactions as
metathesis, hydrogenations and carbonylations. 5
References:
- A. A. N. Magro, L. Robb, P. J. Pogorzelec, A.
M. Z.
Slawin, G. R. Eastman and D. J. Cole-Hamilton,
2010,
1(6),
723-730.
- C.
Jimenez-Rodriguez, Graham R. Eastham and D. J. Cole-Hamilton, Inorg. Chem. Commun.,
2005, 8, 878-881.
- A. A. Núñez Magro, G. R. Eastham and D. J.
Cole-Hamilton,
Chem.
Commun,
2007, 3154-6.
- S. L. Desset and D. J. Cole-Hamilton,
Angew.
Chem.
Int. Ed., 2009, 48,
1472-1474.
- R.
Duque, E. Ochsner, H. Clavier, F. Caijo, S. P. Nolan, M. Mauduit and D.
J. Cole-Hamilton, Green
Chem.,
2011, 13, 1187-1195.
|