Conservation
of archaeological material is
dependent largely upon one factor - enough public interest to pay the
bills. Wetland and marine sites preserve artefacts that are lost on
terrestrial sites, so there is a lot more for people to get interested
in. Unfortunately, these artefacts are difficult to conserve, and this
results in a high cost spread over a long period of time.
Much of the
material we are trying to conserve has
been preserved in its waterlogged condition for longer than museums
have existed. If part of a conservator's role is to preserve this
material, then we must ask whether or not it would be better left where
it is...
In these pages we will be examining some of the
alternatives to conservation. The two main possibilities are reburial
of
material after excavating (Figure 13), and preserving the site in situ.
Ideally
these programs would allow us to preserve cultural heritage, without
the expense of conservation, or risk to the archaeological material. To
do this effectively we must be able to measure how stable the
archaeological material is within a site.
Figure
13. Glastonbury Lake Village - an early reburial experiment
 |
The
excavation of Glastonbury Lake Village
by Arthur Bulleid between 1892 and 1907 was one of the earliest studies
on an English wetland archaeological site. At the time Bulleid
determined that there were no acceptable conservation methods for
waterlogged wood. As a result this material was either reburied, or
stored in water to await the development of better conservation
techniques. The stored material was finally conserved in 1962. Reburial
of material is very common on marine archaeological sites today, due to
the high cost of conservation.
The author's picture of the village shown
above was possible through a reconstruction of its ground plan by J.
Coles and S. Minnit, working from Bulleid's original field notebooks
(J. Coles and S. Minnit, 'Industrious and Fairly Civilised', pub.
Somerset County Council (1995)). |
Further scientific interest
in wreck sites arises
because they represent human impact experiments that have been running
for centuries. Understanding how stable these sites are, and how they
interact with the marine environment may shed light on the ultimate
fate of the material that has been dumped at sea over the last hundred
years. Further pressure for dumping has recently come from the need to
decommission old oil production platforms. Initial plans to dump the
'Brent Spar' in deep water off the continental shelf rightly met with a
storm of opposition. As the original report on this proposal stated, a
study of the deterioration of shipwrecks would have provided an
understanding of the likely environmental impact of this proposal, but
very little work has been done. In the absence of such an understanding
the economically more expensive option of dismantling had to be
adopted. More platforms await decommissioning, the pressure to dump
cheaply grows. The requirement to comprehend human impact on the seas
surrounding us remains. This is a heritage study, which has relevance
to all of us...
Measuring
Preservation Potential
| There are a broad range of factors which
can
influence the preservation environment of a marine archaeological site,
probably the most important is the stability of the sediment cover over
the site. When an artefact is buried it is supported from collapse by
the sediment, protected from damage by storms, from wood eating
organisms like gribble and shipworm, and from looting by divers.
Further, the sediment prevents oxygen reaching the artefacts, so
bacterial and fungal decay processes are slow. As a result the artefact
undergoes slow chemical changes over a period of millennia, but may
remain quite recognisable (Figure 14). From the preservation of
sub-fossil leaves,
we could hypothesise that, under ideal conditions, the artefact could
remain intact for several million years. It is unlikely that the human
race will survive that long! |
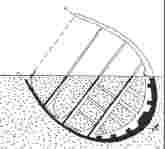
Figure
14. Sketch of a semi-covered artefact
|
Generally, however, we only find marine sites when
they become exposed above the sediment. Exposed sites are subject to
conditions under which rapid decay may be favourable. The questions are
- how rapid, how much of the site is at risk, and what can we do about
it...
Exposure is due to sediments moving in response to
storms, tidal currents, waves and the wakes of ships. Currents can be
localised, giving rise to scour, or only occasionally be strong enough
to cause damage (during storms). In addition, some sediment movement is
under the influence of gravity, for example, settlement and turbidity
currents. Biological organisms, 'worms', squat lobsters and other
creatures will also tunnel or burrow into the sediment about sites, and
this activity will be independent of water currents.
Clearly current measurement alone cannot determine
how stable a sediment is, and large numbers of meters would have to be
deployed over a long time to look at this parameter. Such a study might
be feasible on a single test site, but would not be a practical
technique in the field for determining the risk of deterioration on any
given site. As a result, we intend to look at the problem in a slightly
different way...
Remember we are not interested in the physics of
sediment movement, what is important is - how effectively is the
sediment protecting the site?
Sediments not only physically protect a site, they
also trap seawater within their porous structures. As this water is
separated from open seawater its chemistry will change due to a range
of diagenetic and biological processes. When the sediment is stirred
up, exposing the site, the interstitial water will be replaced with
seawater. A useful measure of how stable the sediment is about the site
may, therefore, be how great the difference is between the seawater and
the water trapped within the pores of the sediment.
If this is true, our problem to find an easily
measured chemical variable, and determine how quickly it changes when
it is trapped within the sediment.
As a result one of our goals will be to develop a
sensor which can be inserted into the sediment and give direct readings
of how long ago the seawater within that sediment was trapped there. In
practice, clearly, there will be a lot of problems...
What types of
parameter will change?
Dissolved oxygen concentration is the most obvious
parameter, as bugs eat the artefact and other entrapped organic matter,
the oxygen within the seawater will be depleted. As a result of this,
the entire redox chemistry of the water within the sediment will
change. Appropriate probes may be simple redox electrodes or more
complex oxygen sensors.
Another important equilibrium process that will be
upset by entrapment within the sediment is that of the CO
2/bicarbonate/carbonate
system. Simplistically this is the inverse of the oxygen concentration
gradient, CO
2 being
produced by the metabolic processes of the bugs within the sediment as
they use up the oxygen. Changes in this equilibrium will change the pH
of the water in the sediment, and pH is more easily measured than
dissolved oxygen.
Finally, there are chemicals released from the
artefacts themselves, metal ions released as a result of corrosion,
organic breakdown products as wood and other material is metabolised on
the site.
It is our intention to look at all of these
parameters, and pick out the most reliable indicators for determining
the stability and preservation of the site.
Problems with
iron wrecks
The above discussion is general to any marine site
- wooden sailing ship, submerged landscape or super tanker. Steel
vessels, or wooden vessels carrying large iron artefacts such as
armaments, however, pose some special problems and opportunities.
Wooden vessels, if not buried in sediment, will normally collapse as
the iron fastenings rust away, leaving only a scattering of guns and
perhaps some heavy keel timbers on the surface to mark the location of
the site. Exposed smaller timbers and light artefacts may become
scattered over quite large areas through the action of currents.
Iron and steel hulled vessels are more recent, so
whilst the metal from which they are made is likely to disappear
eventually due to corrosion, they are frequently in good shape at the
present time. It is apparent that burial again helps to preserve these
wrecks by limiting oxygen access to fuel corrosion, so again the
generic tests above will aid our determining the rate of deterioration.
In addition, however, there are well known techniques of cathodic
protection that might be used to prevent further decay - at
a cost.
Because corrosion is such a major economic
problem, methods have been developed to estimate its rate and likely
impact on modern structures. A direct measurement of the corrosion rate
on archaeological iron could be compared to the known age of the vessel
and or the depth of corrosion product on the item. This would tell us
if corrosion has speeded up, indicating that the site has recently
become less stable.
In short, it may be possible to 'ask' the artefact
whether or not it is OK!
Preliminary studies on this topic are being
carried out at
Cellardyke
pool, close to St Andrews. This pool has been set up by
members of the Scottish Institute of Maritime Studies and the
Archaeological Diving Unit (both at St Andrews) as a site for advanced
training for the Nautical Archaeology Society
Equipment
for Use in In-Situ Conservation Studies
Phase 1 - Equipment development
In order to examine the archaeological site we
must be able to take accurate measurements of the important chemical
variables. This process is very much more difficult underwater than it
would be in the laboratory. The first stage of this project is to
design or adapt equipment to allow measurements to be taken.
Voltages are the simplest parameters to measure
and record. These measurements can be carried out using standard
equipment - digital voltmeters (DVM's) or data loggers. Data loggers
record data, and can be left on site to take measurements when diving
is not possible due to tidal currents or storms.
There are a large number of chemical sensors that
can be used in conjunction with voltmeters, pH probes, corrosion
potential probes, and selective probes for a wide range of analytes. In
addition, simple current and temperature sensors can also be added,
allowing us to determine important physical parameters.
One of the greatest challenges is to armour this
equipment to survive the marine environment, and yet be simple enough
for a diver to use easily. Examples of equipment that we have modified
for marine work are shown below.
Diver
operated equipment
| Some equipment, such as the in situ
monitor (ISM - Figure 15) has been modified at the University of St
Andrews to allow a diver to carry out measurements underwater during
the course of the dive. The ISM1 features a small commercial voltmeter
connected to a corrosion potential electrode. The voltmeter is
encapsulated in a polycarbonate cylinder sealed at either end by brass
plates into which 'o' rings have been inserted. The cable from the
electrode is sealed to prevent water getting to the electronics. The
equipment is switched on and off by magnetic reed switches, operated by
magnets through the clear case, so there are fewer holes for switches
through which leaks can occur. Polycarbonate is one of the toughest
clear
polymers available, and this equipment has been pressure tested to the
equivalent of 100m depth. |
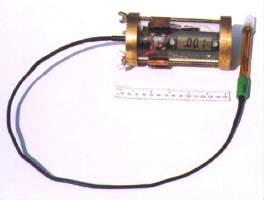 Figure 15. In
Situ Monitor 1 Figure 15. In
Situ Monitor 1
|
Data
logging Equipment
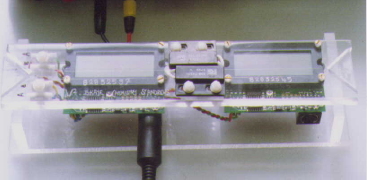
Figure 16. Data Logger V2
|
A diver can use an ISM at any time while
underwater, but it cannot record events that do not occur during the
course of the dive. Hence the need for data logging equipment, like the
V2 (Figure 16, shown out of its polycarbonate shell during
calibration). This features two independent meters that can be attached
to different sensors which are positioned by the diver, who can trigger
the start of the record, again using a magnet, and then leave the V2 in
place to record information over periods of up to one month. |
We
thank Bobby Cathcart
and Brian Walker for their help with the design and construction of
this equipment.
Experimental
Sites
The
evaluation of
deposition sites requires considerable experimental development, we
worked on three sites, Cellardyke
pool is a convenient site close to St Andrews, which allows us to
develop and
test techniques in a relatively sheltered environment. In addition we
were investigating
two shipwreck sites in the Sound of Mull, those of the 'Dartmouth' and
the 'John Preston', in conjunction with the SOMAP project directed by
Phillip Robertson and Steve Webster. The first phase study involves
inserting test samples around the wreck, which will allow us to
determine how aggressive the environment is at these sites.
Cellardyke
Cellardyke bathing pool was
built in the 1930's, largely by the volunteer work of the people of
Cellardyke and Anstruther. The pool is an sheltered water body that
offers a safe location for studies that can be carried out in all but
the worst weather. The water in the pool is replenished each high tide,
and the pool itself has been colonised by an array of sea life, being a
nursery for hermit crabs in spring, and a refuge for many other species
of animal and seaweed.
Figure 17.
Panoramic view of Cellardyke pool today
Several years ago, members
of the Archaeological Diving Unit and the Scottish Institute of
Maritime Studies at St Andrews introduced four cast iron cannons,
dating from the 19th century, into the pool. These cannons had been
recovered in dredging operations. In the pool the guns are protected
from deteriorating in air, and form the basis of a reburial experiment.
The ease of access to the guns allows their condition can be checked
regularly. Today the pool and the guns it encloses provide an ideal
opportunity for divers to learn how to survey underwater, and acquire
some of the skills and qualifications required of the marine
archaeologist.
The ordnance and the
sediments at the bottom of Cellardyke pool provide an ideal site for
developing equipment both for corrosion rate studies, and evaluating
sedimentary characteristics that favour the preservation of marine
archaeology. Unfortunately, the visibility in the pool is usually very
poor (reflecting that on many British marine archaeological sites), so
you wont see many underwater photos here.
Cellardyke pool is itself a
structure of considerable historic interest, the coast of Fife is
dotted by other examples of seawater bathing pools, but many have
suffered breaches to their retaining walls, or have been damaged by
subsequent construction work. Gradually Cellardyke pool is being broken
up by storm wave action, damage extends beyond the coastal defence wall
to the old seating area behind the pool.
Figure 18.
Sketch map of Cellardyke pool
More detailed maps show the
amount of damage to the pool that has occurred since maintenance
stopped some years ago, but are laborious to prepare. Mapping
techniques we are using include baseline offset (which is cheap,
requiring only a couple of tape measures) and plane table surveys,
which allow us to add detail to the map. We have also used an
Electronic Distance Measurement (EDM) system.
 |
 |
 |
Figure 19. Students
using the EDM at Cellardyke
|
Figure 20. Putting the web of points
produced with the EDM over the plane table data
|
Figure 21. It
can be cold, wet, and windy work
|

Figure 21. Photograph of damage to the
concrete diving platform. Slabs of reinforced concrete six inches thick
have been lifted and broken by the action of storm waves
Acknowledgements
Due to lack of funding, the
in situ conservation studies have had to be limited to what can be
achieved by a small group of enthusiasts in their spare time. My thanks
go out to all the members of St Andrews Archaeological Divers for their
help, without these people non-of this work would have been possible.
Further thanks to students of the Scottish Institute of Maritime
Studies, and the Geology Department at the University.
A history of
Cellardyke
pool is in preparation by Jo Cook, one of Diving Club members.
Ruben Duque 2011.
All rights reserved